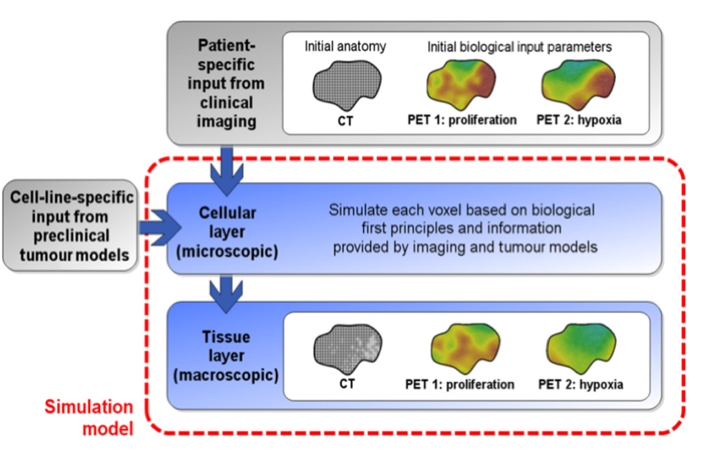
We are modeling tumor progression based on imaging inputs to better understand complex relationships leading to tumor heterogeneity and treatment resistance.
- Imaging-based Modeling of Radiation Treatment Response
- Modeling of Tumor and Vasculature Growth
- Modeling Clinical Trial Inclusion
- Multi-scale tumor modeling can derive theoretical solutions to cancer therapy questions by allowing inclusion of patient-specific biological information for modeling and validation of clinical scenarios
- Development of the imaging-based model of tumor growth and response to therapies that allows assessment of realistic clinical scenarios – next generation treatment planning
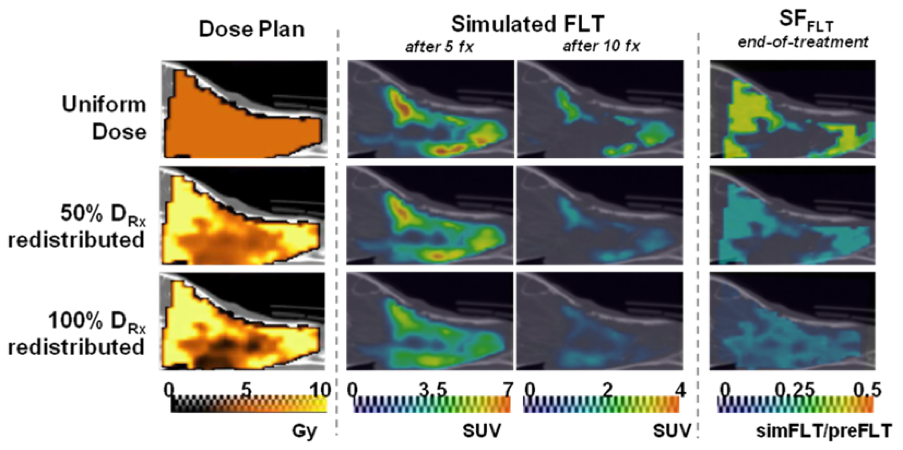
Modeling Application: How much dose to redistribute in dose painting?
- Dose prescription function:
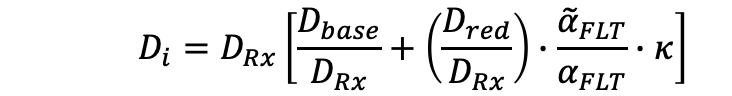
- Increased uniformity of response with increased redistribution
- Future: Optimization of dose painting strategies in humans
Modeling Application: How much dose to redistribute in dose painting?
Titz and Jeraj, Phys Med Biol 2008
- Modeling based on imaging data as an extremely versatile tool for analyzing individual tumors and their progression
- Development of the vasculature based models have been developed demonstrating tumor growth and simulation of micro-environmental characteristics
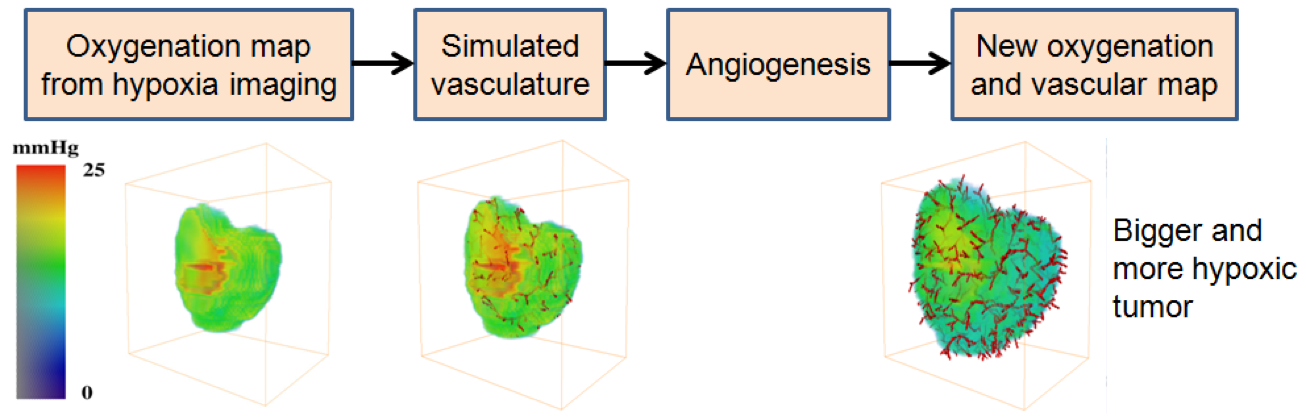
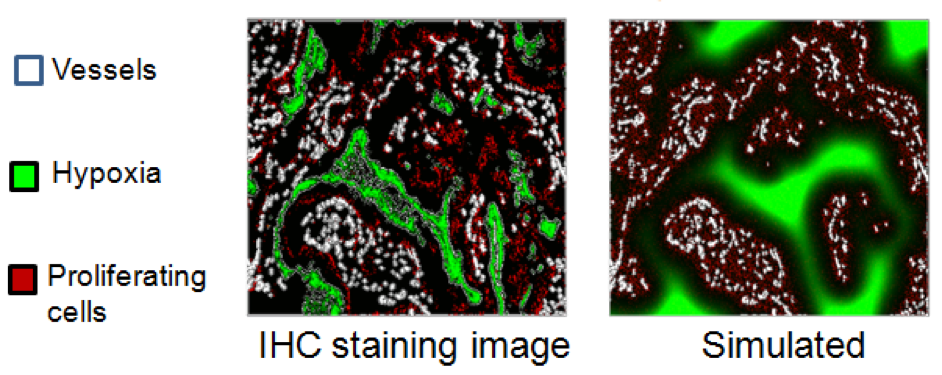
- Vasculature data can also be used to replicate hypoxia patterns and proliferative cell density for benchmarking and improvement
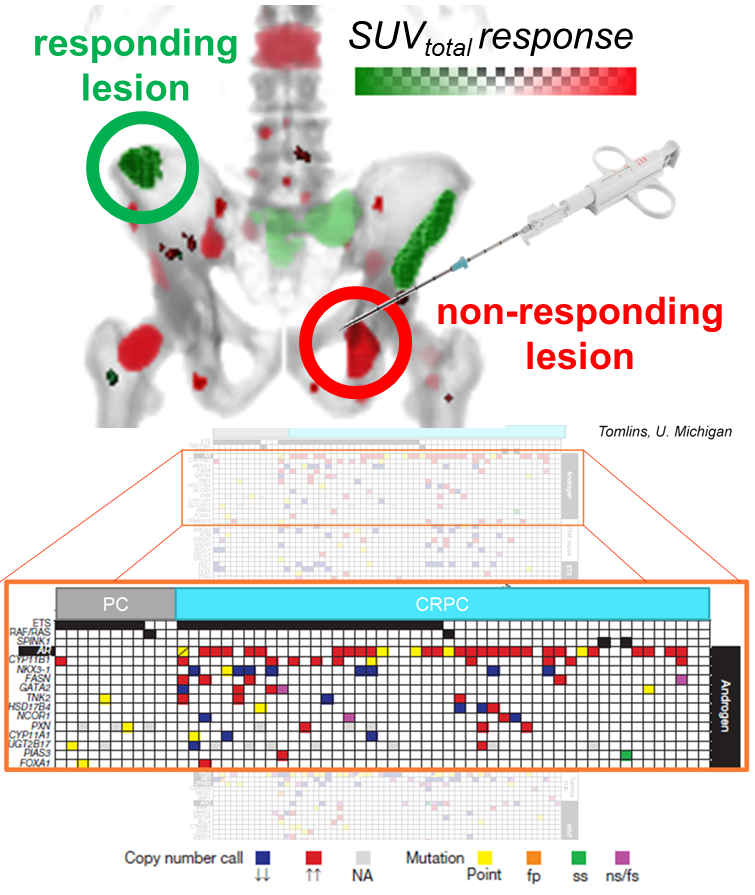
- Evaluate imaging biomarkers’ prognostic value for patient outcome (Responders vs. Non-responders
- Future: Incorporation of mechanisms of treatment resistance
Titz et al, Phys Med Biol 2012
Adhikarla and Jeraj, Phys Med Biol 2012
- Clinical trial patient inclusion is traditionally based on patient characteristics such as diagnosis of metastatic cancer through imaging or PSA level, but for our MIB clinical trial additional lesion-level characteristics need taken into consideration, specifically how much metastatic disease is present
- The purpose of this study is to improve clinical trial patient selection by determining the probability of a patient having suitable lesions at the time of biopsy based on a previous clinical trial
- By sampling an existing, similar population to that of the MIB trial, we can determine probabilistic methods for determining whether or not a future patient is suited for a given trial
- This method allows an initial simulation of a prospective patient population and could improve patient selection criteria for similar clinical trials